Xilis’ rapid and scalable patient-derived MicroOrganoSphereTM (MOS) technology platform enhances precision medicine and pharmaceutical drug discovery and development.
Compared to conventional patient-derived models, MOSTM provides faster, higher-throughput, and greater cost savings, as well as more predictive and accurate results. MOSTM can be used in large-scale pharmacogenomic studies that are representative of the breadth of human genetic diversity during pre-clinical development. We believe the technology not only has the potential to make research more cost efficient, but also significantly more effective.
With ultra high-throughput screening and custom assay capabilities, drug developers can use MOSTM from preclinical studies to proof of concept clinical trials. We believe this approach provides the highest probability of success for our collaboration partners and is the right way to create value for them, for Xilis, and most importantly, for patients.
Choosing the Right Patient Populations
Personalized medicines in immuno-oncology and infectious disease are crucial to ensure patients get the most effective treatment regimen. The MOSTM technology enables drug targeting for specific populations. Following tissue biopsy and generation of patient-derived MOSTM, researchers and clinicians can predict which patient populations will benefit most from a specific drug. By incorporating this step, Xilis’ MOSTM technology can help reduce time and costs at all stages of the drug development process, from lead generation through clinical studies.
To learn more about MOSTM and Precision Oncology, click here
Cancers
- Anal
- Bladder
- Brain
- Breast
- Colon
- Esophagus
- Head & neck
- Gastric
- Liver
- Lung
- Kidney
- Melanoma
- Mesothelioma
- Neuroendocrine
- Ovary
- Prostate
- Sarcoma
- Uterus
Healthy Tissue
- Brain
- Colon
- Airway
- Lung
- Esophagus
- Heart
- Intestine
- Kidney
- Liver
- Nasal epithelium
- Olfactory bulb
- Skin
- Stomach
- Testicle
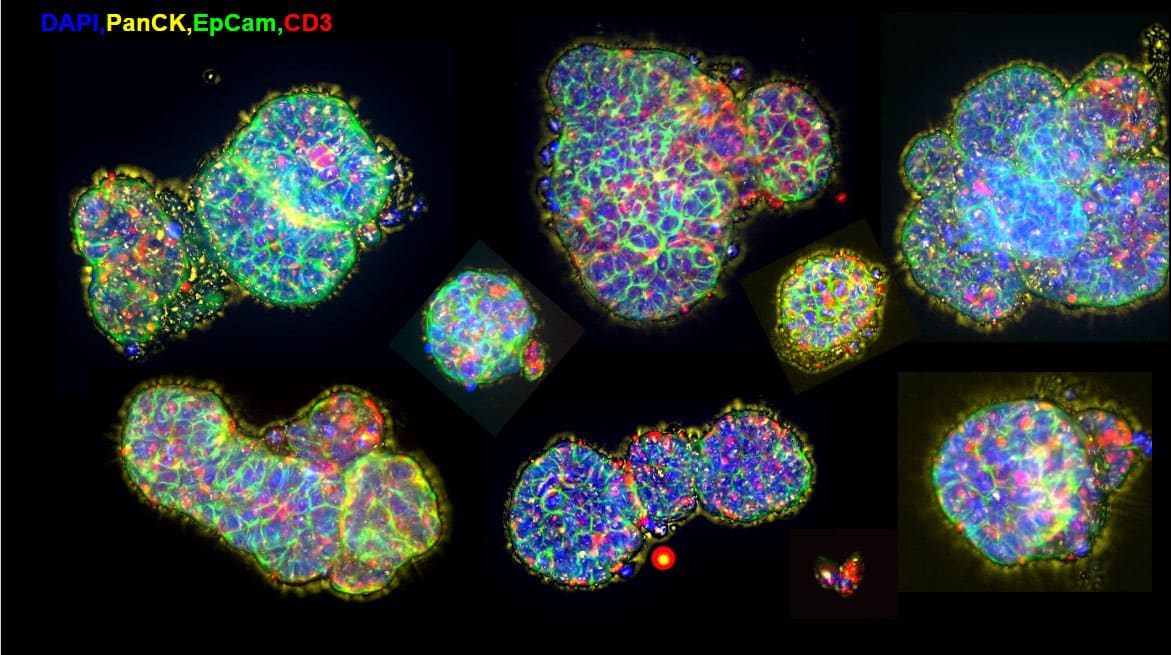
Validation in Solid Tumors and Healthy Tissues
MOSTM has been established and validated in both cancer and healthy tissues. With a high establishment rate, Xilis’ technology allows for the handling of complex samples that can generate usable models from multiple primary tissue types. This helps drug developers determine both safety and efficacy of a specific drug in diseased or normal conditions. Xilis’ MOSTM ATLAS -- a compilation of MOSTM from a wide breadth of organs and patients -- can be used for robust, rapid and efficient pharmacogenetic studies at scale.
MOSTM Functionality
MOSTM enables researchers to develop assays that test functionality. Shown in the figure are hepatocytes derived from a patient’s liver. The hepatocyte MOSTM remains functional after establishment as demonstrated by internalization of LDL from the media.
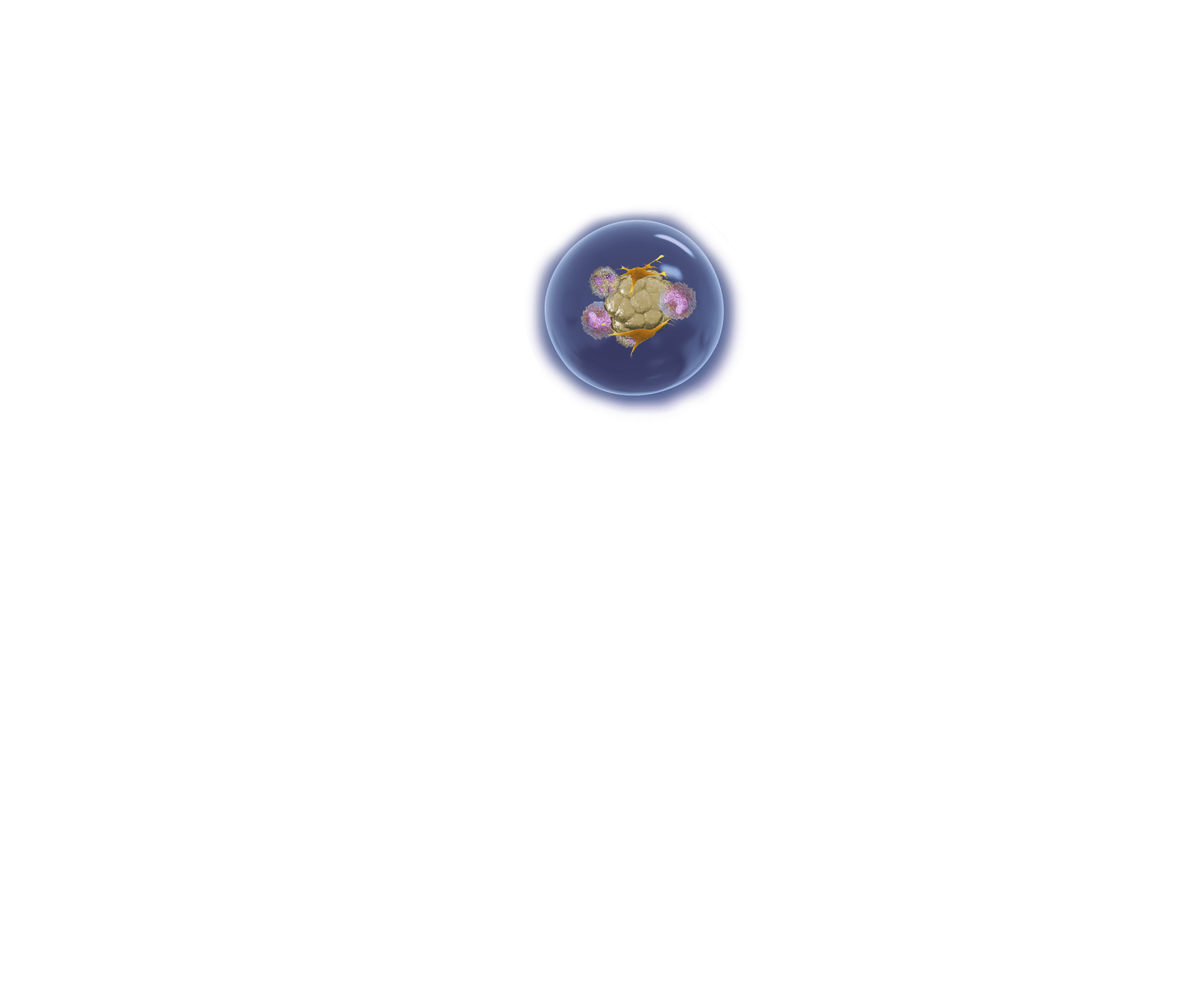
Immuno-oncology Drug Development
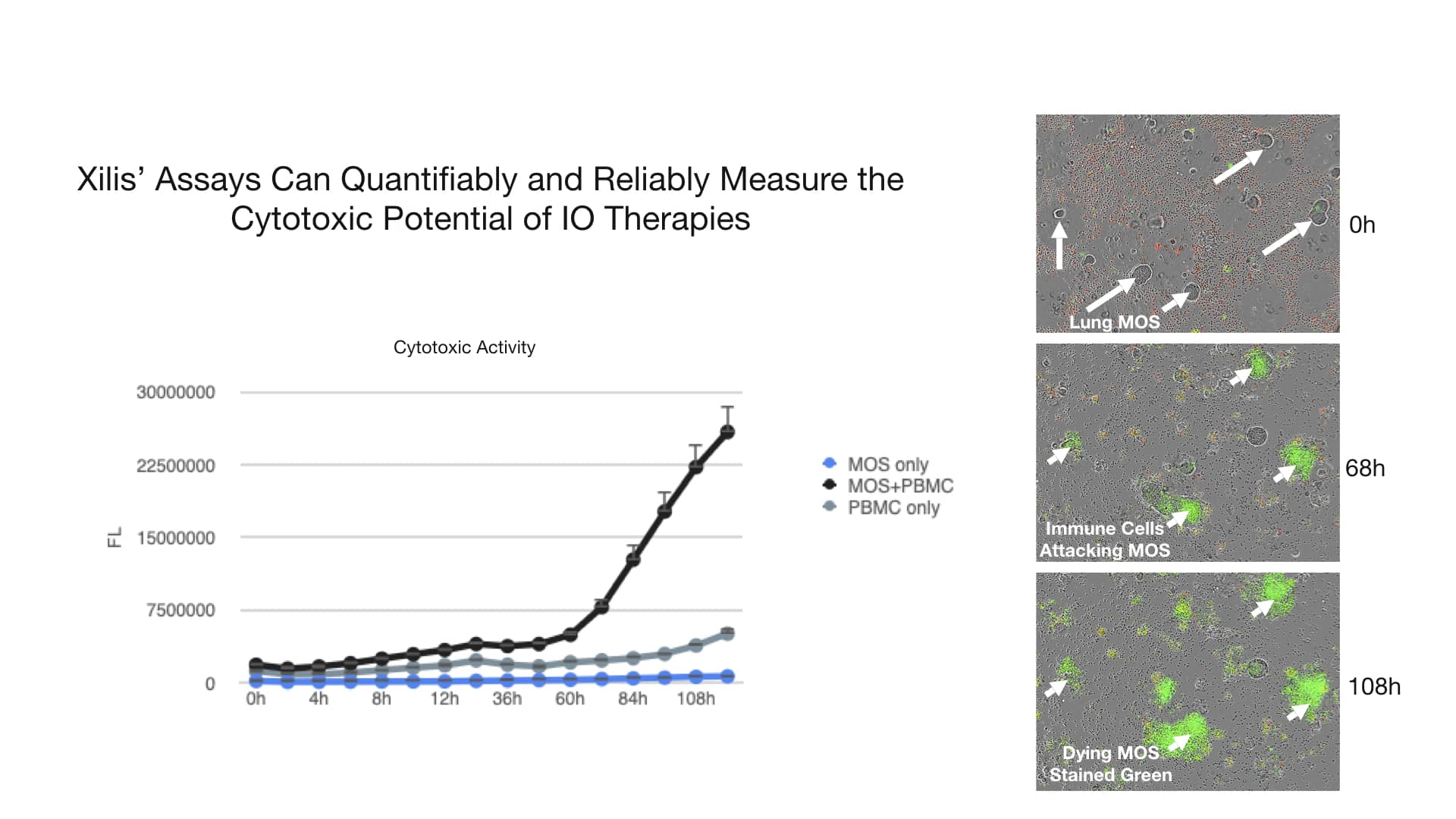
Xilis’ platform provides drug developers an innovative technique to evaluate immuno-oncology therapeutic candidates. Since each MOSTM captures the full microenvironment and cellular heterogeneity of the patient’s tumor, our high-throughput platform can be used to investigate how single agents or combination therapies induce the immune system response and its ability to kill tumor cells. For example, lung cancer MOSTM are grown and established. T-cells are then added and activated. The T-cells attack the MOSTM. Green dye (Caspase3/7) is used to label dying cells, demonstrating the cytotoxic effect T-cells have on MOSTM.
The graph on the right quantifies the fluorescence intensity, making it a quantitative assay.
Pharmaceutical companies can use Xilis’ platform to investigate immunotherapeutic candidates (e.g. checkpoint inhibitors, CAR-T, TILs) as our MOSTM captures the full tumor microenvironment. By using our technology, drug developers can increase clinical trial success rates by ensuring drugs are safe and effective, and to target patients most likely to respond to treatment.